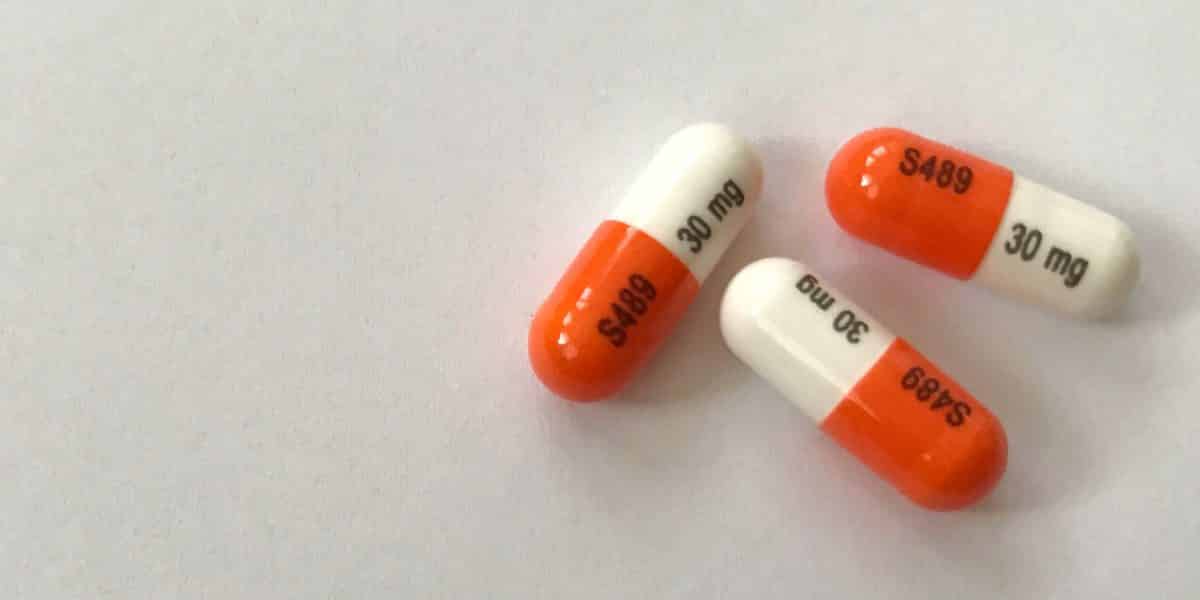
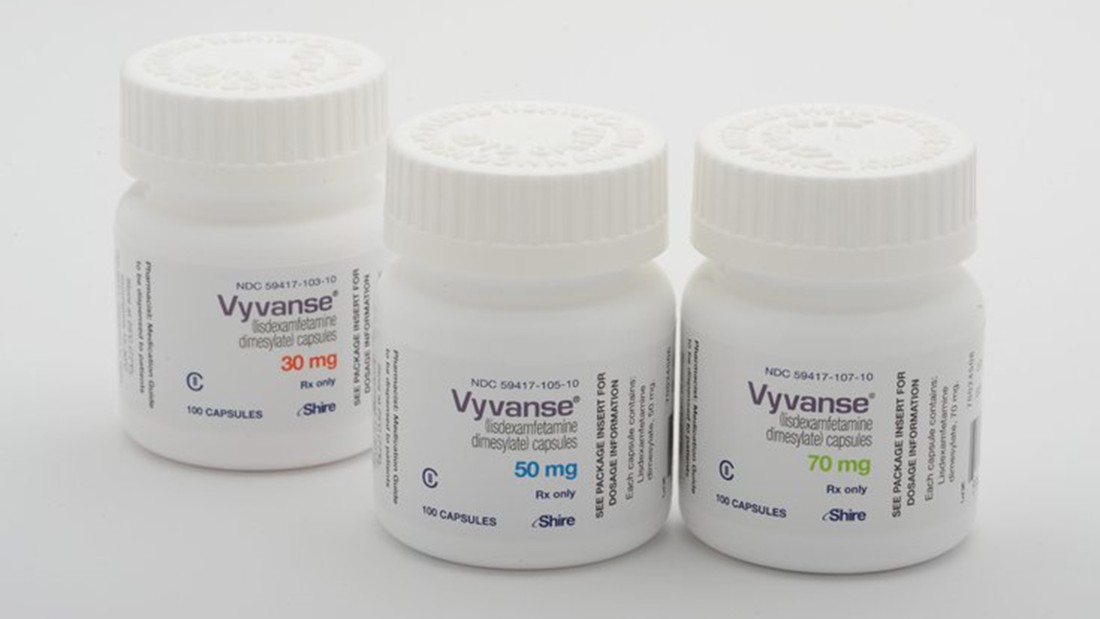
Generic Vyvanse Availability
Generic Vyvanse Availability - The new vyvanse generic products hit the market in august 2023, shortly after the u.s. The fda approved the generic version of vyvanse (lisdexamfetamine) on august 28, 2023, and began to hit the shelves shortly.
The new vyvanse generic products hit the market in august 2023, shortly after the u.s. The fda approved the generic version of vyvanse (lisdexamfetamine) on august 28, 2023, and began to hit the shelves shortly.
The fda approved the generic version of vyvanse (lisdexamfetamine) on august 28, 2023, and began to hit the shelves shortly. The new vyvanse generic products hit the market in august 2023, shortly after the u.s.
Vyvanse Dosage Adults a Quick Rundown MEDvidi
The new vyvanse generic products hit the market in august 2023, shortly after the u.s. The fda approved the generic version of vyvanse (lisdexamfetamine) on august 28, 2023, and began to hit the shelves shortly.
Vyvanse Addiction Treatment The Recovery Village Ridgefield
The fda approved the generic version of vyvanse (lisdexamfetamine) on august 28, 2023, and began to hit the shelves shortly. The new vyvanse generic products hit the market in august 2023, shortly after the u.s.
Signs Your Vyvanse Dose is too High Mandala Healing Center
The new vyvanse generic products hit the market in august 2023, shortly after the u.s. The fda approved the generic version of vyvanse (lisdexamfetamine) on august 28, 2023, and began to hit the shelves shortly.
Vyvanse Addiction Treatment & Abuse Warning Signs
The fda approved the generic version of vyvanse (lisdexamfetamine) on august 28, 2023, and began to hit the shelves shortly. The new vyvanse generic products hit the market in august 2023, shortly after the u.s.
FDA Clears Generic Vyvanse as ADHD Medication Shortage Continues
The fda approved the generic version of vyvanse (lisdexamfetamine) on august 28, 2023, and began to hit the shelves shortly. The new vyvanse generic products hit the market in august 2023, shortly after the u.s.
FDA Clears Generic Vyvanse as ADHD Medication Shortage Continues
The fda approved the generic version of vyvanse (lisdexamfetamine) on august 28, 2023, and began to hit the shelves shortly. The new vyvanse generic products hit the market in august 2023, shortly after the u.s.
ADD/ ADHD Medications with stimulants Vyvanse (Lisdexamfetamine)
The fda approved the generic version of vyvanse (lisdexamfetamine) on august 28, 2023, and began to hit the shelves shortly. The new vyvanse generic products hit the market in august 2023, shortly after the u.s.
ADD/ ADHD Medications with stimulants Vyvanse (Lisdexamfetamine)
The fda approved the generic version of vyvanse (lisdexamfetamine) on august 28, 2023, and began to hit the shelves shortly. The new vyvanse generic products hit the market in august 2023, shortly after the u.s.
Vyvanse for ADHD Ballard Psychiatry
The new vyvanse generic products hit the market in august 2023, shortly after the u.s. The fda approved the generic version of vyvanse (lisdexamfetamine) on august 28, 2023, and began to hit the shelves shortly.
The Fda Approved The Generic Version Of Vyvanse (Lisdexamfetamine) On August 28, 2023, And Began To Hit The Shelves Shortly.
The new vyvanse generic products hit the market in august 2023, shortly after the u.s.