How Many Valence Electrons Are Available For Bonding In Silicon
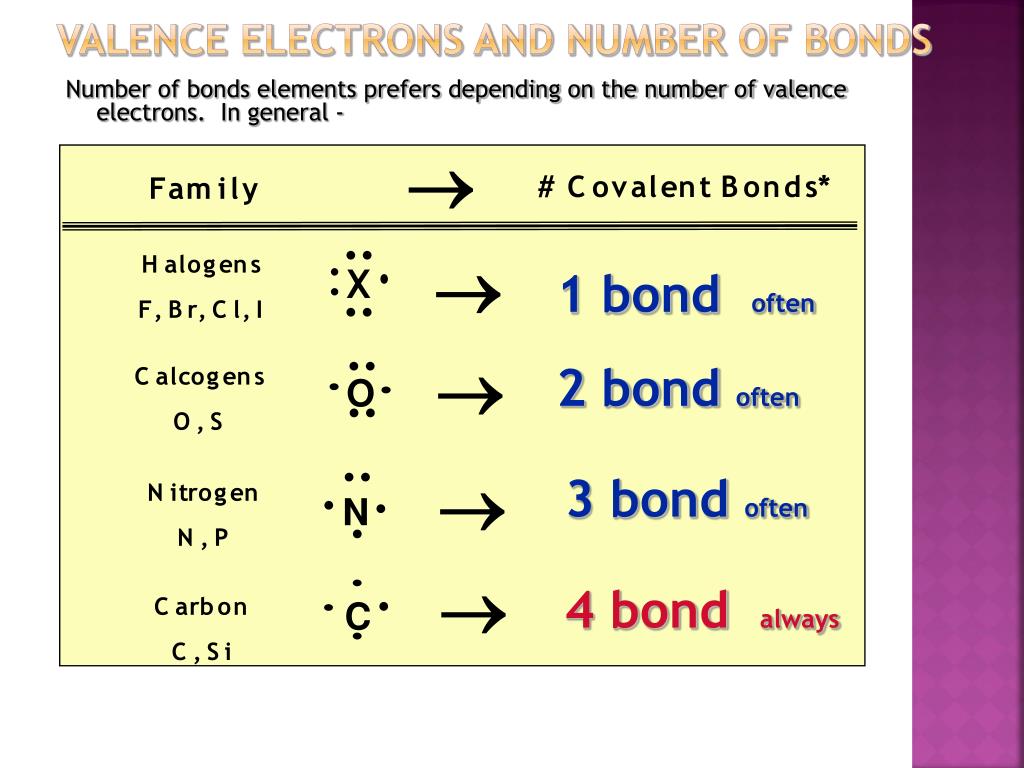
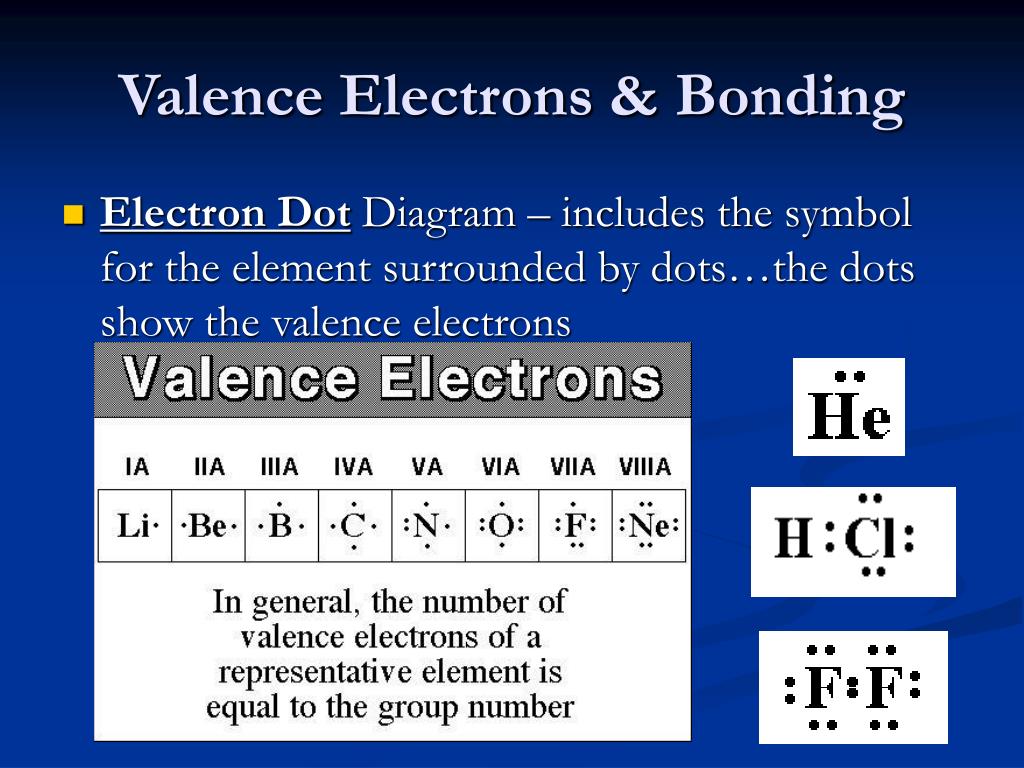
How Many Valence Electrons Are Available For Bonding In Silicon - The four valence electrons allow silicon to form covalent bonds, which are essential in many materials, including semiconductors and silicon. Silicon, a very common substance with an atomic number of 14, has four valence electrons available for bonding. About 1022 atoms in a cubic centimeter of about 1022 atoms in a cubic centimeter of crystal, but there are only about 1010 /cm3 free electrons. This electron configuration is crucial because the electrons in the outermost shell (the valence shell) dictate how silicon interacts with other.
Silicon, a very common substance with an atomic number of 14, has four valence electrons available for bonding. The four valence electrons allow silicon to form covalent bonds, which are essential in many materials, including semiconductors and silicon. This electron configuration is crucial because the electrons in the outermost shell (the valence shell) dictate how silicon interacts with other. About 1022 atoms in a cubic centimeter of about 1022 atoms in a cubic centimeter of crystal, but there are only about 1010 /cm3 free electrons.
Silicon, a very common substance with an atomic number of 14, has four valence electrons available for bonding. About 1022 atoms in a cubic centimeter of about 1022 atoms in a cubic centimeter of crystal, but there are only about 1010 /cm3 free electrons. This electron configuration is crucial because the electrons in the outermost shell (the valence shell) dictate how silicon interacts with other. The four valence electrons allow silicon to form covalent bonds, which are essential in many materials, including semiconductors and silicon.
How Many Valence Electrons Does Silicon (Si) Have?
This electron configuration is crucial because the electrons in the outermost shell (the valence shell) dictate how silicon interacts with other. The four valence electrons allow silicon to form covalent bonds, which are essential in many materials, including semiconductors and silicon. Silicon, a very common substance with an atomic number of 14, has four valence electrons available for bonding. About.
How Many Valence Electrons Does Silicon(Si) Have?
About 1022 atoms in a cubic centimeter of about 1022 atoms in a cubic centimeter of crystal, but there are only about 1010 /cm3 free electrons. The four valence electrons allow silicon to form covalent bonds, which are essential in many materials, including semiconductors and silicon. This electron configuration is crucial because the electrons in the outermost shell (the valence.
PPT Bonding PowerPoint Presentation, free download ID6281432
The four valence electrons allow silicon to form covalent bonds, which are essential in many materials, including semiconductors and silicon. This electron configuration is crucial because the electrons in the outermost shell (the valence shell) dictate how silicon interacts with other. About 1022 atoms in a cubic centimeter of about 1022 atoms in a cubic centimeter of crystal, but there.
PPT Chapter 5 Atoms & Bonding PowerPoint Presentation, free
This electron configuration is crucial because the electrons in the outermost shell (the valence shell) dictate how silicon interacts with other. About 1022 atoms in a cubic centimeter of about 1022 atoms in a cubic centimeter of crystal, but there are only about 1010 /cm3 free electrons. The four valence electrons allow silicon to form covalent bonds, which are essential.
Number of valence electrons in silicon
About 1022 atoms in a cubic centimeter of about 1022 atoms in a cubic centimeter of crystal, but there are only about 1010 /cm3 free electrons. Silicon, a very common substance with an atomic number of 14, has four valence electrons available for bonding. The four valence electrons allow silicon to form covalent bonds, which are essential in many materials,.
What is Silicon Electron Configuration Understanding the Atomic
The four valence electrons allow silicon to form covalent bonds, which are essential in many materials, including semiconductors and silicon. This electron configuration is crucial because the electrons in the outermost shell (the valence shell) dictate how silicon interacts with other. Silicon, a very common substance with an atomic number of 14, has four valence electrons available for bonding. About.
PPT Chemical Bonding and Molecular Structure PowerPoint Presentation
About 1022 atoms in a cubic centimeter of about 1022 atoms in a cubic centimeter of crystal, but there are only about 1010 /cm3 free electrons. Silicon, a very common substance with an atomic number of 14, has four valence electrons available for bonding. This electron configuration is crucial because the electrons in the outermost shell (the valence shell) dictate.
how many valence electrons are in a silicon atom
About 1022 atoms in a cubic centimeter of about 1022 atoms in a cubic centimeter of crystal, but there are only about 1010 /cm3 free electrons. This electron configuration is crucial because the electrons in the outermost shell (the valence shell) dictate how silicon interacts with other. Silicon, a very common substance with an atomic number of 14, has four.
Introduction to Electronics Study Guides CircuitBread
About 1022 atoms in a cubic centimeter of about 1022 atoms in a cubic centimeter of crystal, but there are only about 1010 /cm3 free electrons. This electron configuration is crucial because the electrons in the outermost shell (the valence shell) dictate how silicon interacts with other. The four valence electrons allow silicon to form covalent bonds, which are essential.
Bonding and Chemical Equations ppt download
Silicon, a very common substance with an atomic number of 14, has four valence electrons available for bonding. The four valence electrons allow silicon to form covalent bonds, which are essential in many materials, including semiconductors and silicon. This electron configuration is crucial because the electrons in the outermost shell (the valence shell) dictate how silicon interacts with other. About.
This Electron Configuration Is Crucial Because The Electrons In The Outermost Shell (The Valence Shell) Dictate How Silicon Interacts With Other.
Silicon, a very common substance with an atomic number of 14, has four valence electrons available for bonding. The four valence electrons allow silicon to form covalent bonds, which are essential in many materials, including semiconductors and silicon. About 1022 atoms in a cubic centimeter of about 1022 atoms in a cubic centimeter of crystal, but there are only about 1010 /cm3 free electrons.