Is Neffy Available
Is Neffy Available - Neffy is expected to be available in the united states within eight weeks of fda approval for patients who weigh 30 kg or. Food and drug administration approved neffy (epinephrine nasal spray) for the emergency treatment of allergic reactions.
Food and drug administration approved neffy (epinephrine nasal spray) for the emergency treatment of allergic reactions. Neffy is expected to be available in the united states within eight weeks of fda approval for patients who weigh 30 kg or.
Neffy is expected to be available in the united states within eight weeks of fda approval for patients who weigh 30 kg or. Food and drug administration approved neffy (epinephrine nasal spray) for the emergency treatment of allergic reactions.
Neffy Uses, Dosage, Side Effects, Warnings
Neffy is expected to be available in the united states within eight weeks of fda approval for patients who weigh 30 kg or. Food and drug administration approved neffy (epinephrine nasal spray) for the emergency treatment of allergic reactions.
FDA Accepts New Drug Application for Nasal Epinephrine Spray Treatment
Neffy is expected to be available in the united states within eight weeks of fda approval for patients who weigh 30 kg or. Food and drug administration approved neffy (epinephrine nasal spray) for the emergency treatment of allergic reactions.
FDA approves First Nasal Spray for Treatment of Anaphylaxis (neffy
Food and drug administration approved neffy (epinephrine nasal spray) for the emergency treatment of allergic reactions. Neffy is expected to be available in the united states within eight weeks of fda approval for patients who weigh 30 kg or.
Epinephrine Nasal Spray Neffy Now Available to Treat Allergic Reactions
Neffy is expected to be available in the united states within eight weeks of fda approval for patients who weigh 30 kg or. Food and drug administration approved neffy (epinephrine nasal spray) for the emergency treatment of allergic reactions.
What is Neffy? FDA approves first nasal spray to treat severe allergies
Food and drug administration approved neffy (epinephrine nasal spray) for the emergency treatment of allergic reactions. Neffy is expected to be available in the united states within eight weeks of fda approval for patients who weigh 30 kg or.
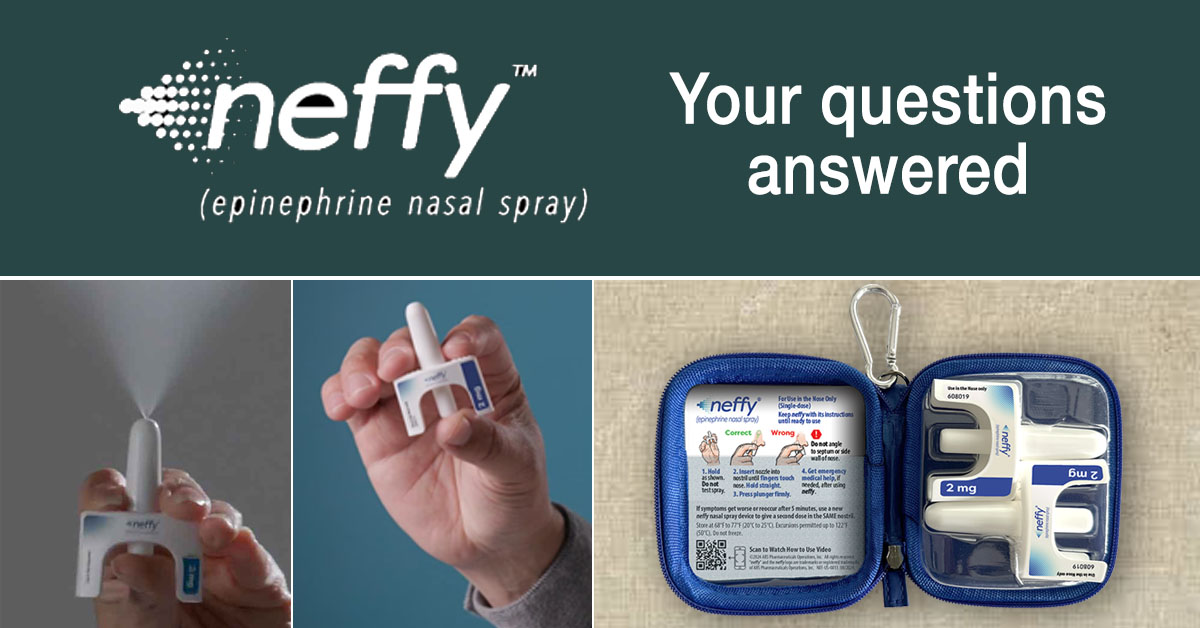
Your neffy Questions Answered
Neffy is expected to be available in the united states within eight weeks of fda approval for patients who weigh 30 kg or. Food and drug administration approved neffy (epinephrine nasal spray) for the emergency treatment of allergic reactions.
FDA Approves Neffy First Nasal Spray for Emergency Allergic Reactions
Food and drug administration approved neffy (epinephrine nasal spray) for the emergency treatment of allergic reactions. Neffy is expected to be available in the united states within eight weeks of fda approval for patients who weigh 30 kg or.
Neffy Is About to Hit Shelves Considerations for Prescribing MedPage
Neffy is expected to be available in the united states within eight weeks of fda approval for patients who weigh 30 kg or. Food and drug administration approved neffy (epinephrine nasal spray) for the emergency treatment of allergic reactions.
Neffy 1 mg now available for anaphylaxis in children aged 4 years and up
Neffy is expected to be available in the united states within eight weeks of fda approval for patients who weigh 30 kg or. Food and drug administration approved neffy (epinephrine nasal spray) for the emergency treatment of allergic reactions.
Neffy Is Expected To Be Available In The United States Within Eight Weeks Of Fda Approval For Patients Who Weigh 30 Kg Or.
Food and drug administration approved neffy (epinephrine nasal spray) for the emergency treatment of allergic reactions.