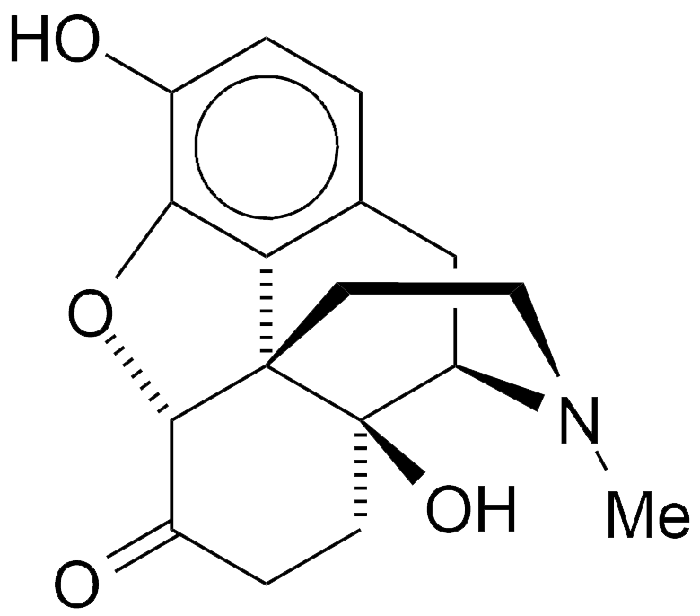
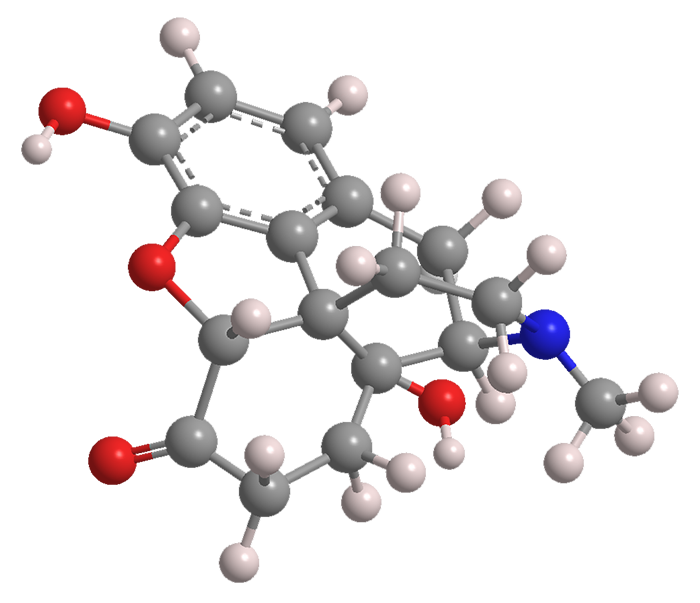
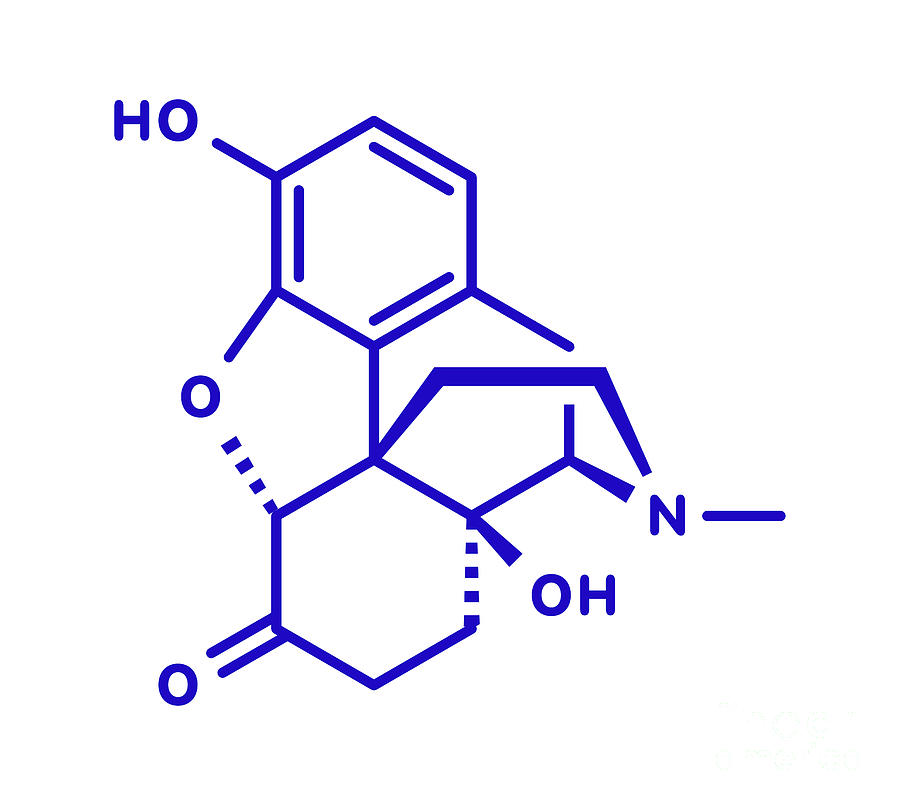
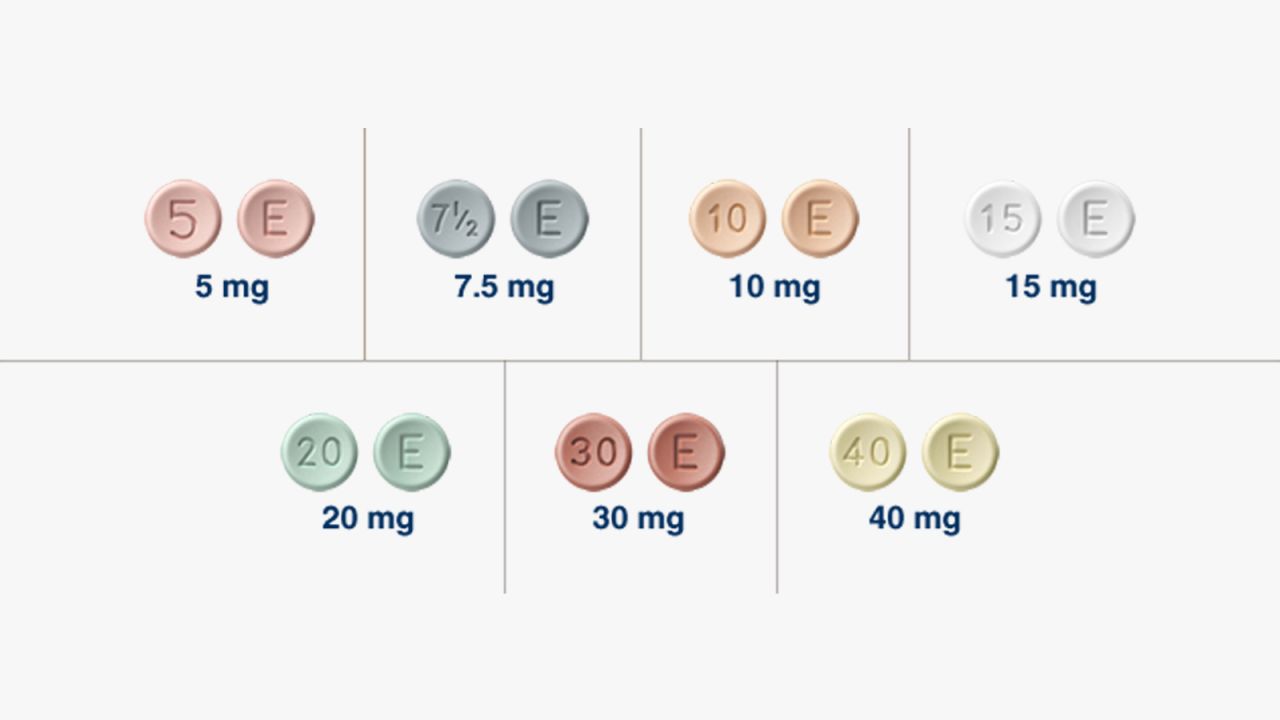
Is Oxymorphone Still Available
Is Oxymorphone Still Available - **not currently available or marketed due to a voluntary recall, but is still approved. Oxymorphone has a high potential for addiction, abuse, and misuse similar to morphine and can lead to overdose and death. Recently generic oxymorphone formulations were approved by fda. However, opana er® was discontinued in 2017.
Oxymorphone has a high potential for addiction, abuse, and misuse similar to morphine and can lead to overdose and death. However, opana er® was discontinued in 2017. Recently generic oxymorphone formulations were approved by fda. **not currently available or marketed due to a voluntary recall, but is still approved.
Recently generic oxymorphone formulations were approved by fda. **not currently available or marketed due to a voluntary recall, but is still approved. Oxymorphone has a high potential for addiction, abuse, and misuse similar to morphine and can lead to overdose and death. However, opana er® was discontinued in 2017.
How Long Does Opana (Oxymorphone) Withdrawal Last?
**not currently available or marketed due to a voluntary recall, but is still approved. However, opana er® was discontinued in 2017. Oxymorphone has a high potential for addiction, abuse, and misuse similar to morphine and can lead to overdose and death. Recently generic oxymorphone formulations were approved by fda.
Oxymorphone American Chemical Society
However, opana er® was discontinued in 2017. Recently generic oxymorphone formulations were approved by fda. Oxymorphone has a high potential for addiction, abuse, and misuse similar to morphine and can lead to overdose and death. **not currently available or marketed due to a voluntary recall, but is still approved.
Opana (Oxymorphone) Side Effects, Interactions, Uses, Dosage, Warnings
Oxymorphone has a high potential for addiction, abuse, and misuse similar to morphine and can lead to overdose and death. However, opana er® was discontinued in 2017. **not currently available or marketed due to a voluntary recall, but is still approved. Recently generic oxymorphone formulations were approved by fda.
Opana (Oxymorphone) Side Effects, Interactions, Uses, Dosage, Warnings
However, opana er® was discontinued in 2017. Recently generic oxymorphone formulations were approved by fda. Oxymorphone has a high potential for addiction, abuse, and misuse similar to morphine and can lead to overdose and death. **not currently available or marketed due to a voluntary recall, but is still approved.
Oxymorphone American Chemical Society
Recently generic oxymorphone formulations were approved by fda. **not currently available or marketed due to a voluntary recall, but is still approved. However, opana er® was discontinued in 2017. Oxymorphone has a high potential for addiction, abuse, and misuse similar to morphine and can lead to overdose and death.
Products Buy Oxymorphone from Global RC And Pharmaceuticals Products
Recently generic oxymorphone formulations were approved by fda. Oxymorphone has a high potential for addiction, abuse, and misuse similar to morphine and can lead to overdose and death. **not currently available or marketed due to a voluntary recall, but is still approved. However, opana er® was discontinued in 2017.
Oxymorphone (Opana) Uses, Dose, Side effects, MOA, Brands
However, opana er® was discontinued in 2017. Recently generic oxymorphone formulations were approved by fda. Oxymorphone has a high potential for addiction, abuse, and misuse similar to morphine and can lead to overdose and death. **not currently available or marketed due to a voluntary recall, but is still approved.
Oxymorphone Opioid Analgesic Drug Molecule Photograph by Molekuul
**not currently available or marketed due to a voluntary recall, but is still approved. Oxymorphone has a high potential for addiction, abuse, and misuse similar to morphine and can lead to overdose and death. Recently generic oxymorphone formulations were approved by fda. However, opana er® was discontinued in 2017.
What Is Oxymorphone? Atlanta, GA Retreat of Atlanta
Oxymorphone has a high potential for addiction, abuse, and misuse similar to morphine and can lead to overdose and death. **not currently available or marketed due to a voluntary recall, but is still approved. However, opana er® was discontinued in 2017. Recently generic oxymorphone formulations were approved by fda.
Opioid Crisis Fast Facts CNN
**not currently available or marketed due to a voluntary recall, but is still approved. However, opana er® was discontinued in 2017. Oxymorphone has a high potential for addiction, abuse, and misuse similar to morphine and can lead to overdose and death. Recently generic oxymorphone formulations were approved by fda.
Oxymorphone Has A High Potential For Addiction, Abuse, And Misuse Similar To Morphine And Can Lead To Overdose And Death.
However, opana er® was discontinued in 2017. **not currently available or marketed due to a voluntary recall, but is still approved. Recently generic oxymorphone formulations were approved by fda.