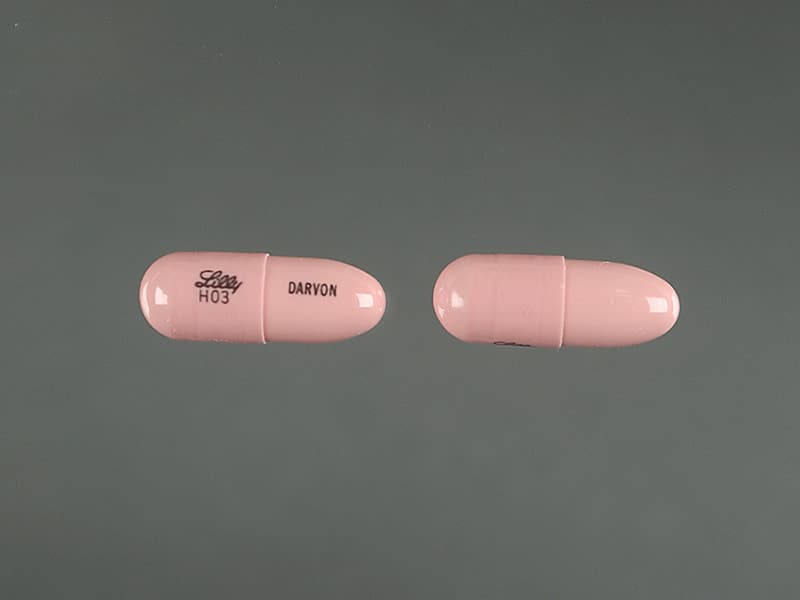
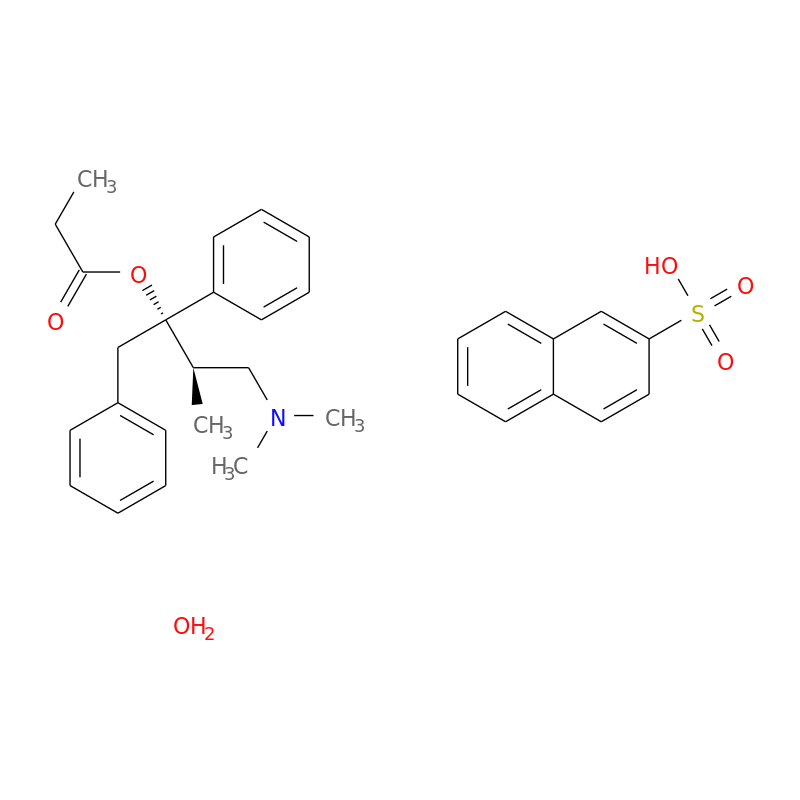
Is Propoxyphene Still Available
Is Propoxyphene Still Available - It was that study that led to the drug being pulled from the market friday. The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. The agency has also asked makers of generic drugs.
The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. The agency has also asked makers of generic drugs. It was that study that led to the drug being pulled from the market friday.
It was that study that led to the drug being pulled from the market friday. The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. The agency has also asked makers of generic drugs.
DailyMed PROPOXYPHENE propoxyphene hydrochloride capsule
The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. The agency has also asked makers of generic drugs. It was that study that led to the drug being pulled from the market friday.
Propoxyphene Drug Test Included in a 10 Panel Drug Test
The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. The agency has also asked makers of generic drugs. It was that study that led to the drug being pulled from the market friday.
OPIATES Mrs.Farina. ppt download
The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. It was that study that led to the drug being pulled from the market friday. The agency has also asked makers of generic drugs.
Propoxyphene SIELC Technologies
The agency has also asked makers of generic drugs. The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. It was that study that led to the drug being pulled from the market friday.
PROPOXYPHENE Generic Name, Drug class, Brande Name ,Precautions
The agency has also asked makers of generic drugs. The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. It was that study that led to the drug being pulled from the market friday.
PPT Pain Management Updates and Issues PowerPoint Presentation, free
It was that study that led to the drug being pulled from the market friday. The agency has also asked makers of generic drugs. The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias.
What is Propoxyphene YouTube
The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. It was that study that led to the drug being pulled from the market friday. The agency has also asked makers of generic drugs.
Propoxyphene brand name list from
The agency has also asked makers of generic drugs. The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. It was that study that led to the drug being pulled from the market friday.
PPT A Comprehensive Review of Treating Acute Pain PowerPoint
The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. The agency has also asked makers of generic drugs. It was that study that led to the drug being pulled from the market friday.
The Agency Has Also Asked Makers Of Generic Drugs.
It was that study that led to the drug being pulled from the market friday. The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias.