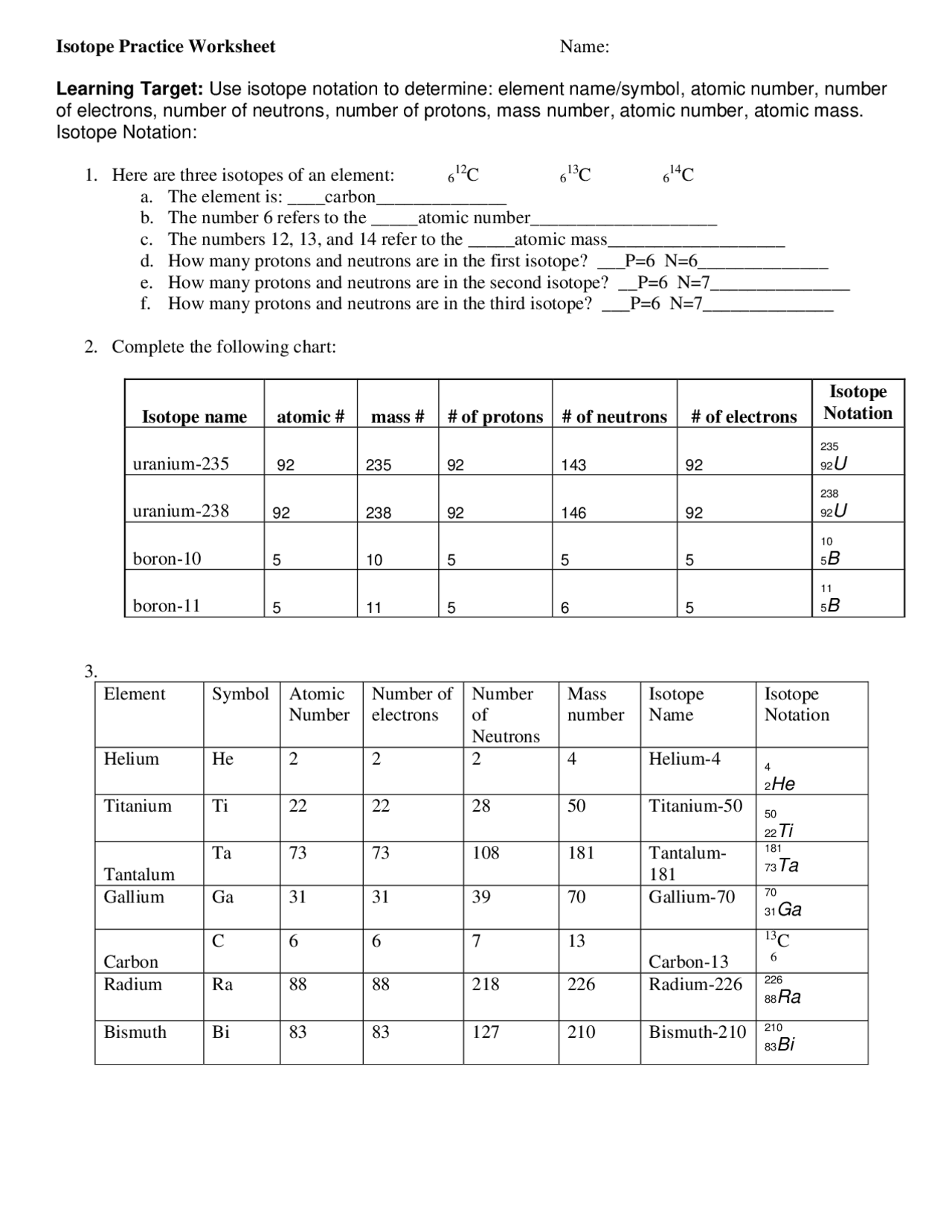
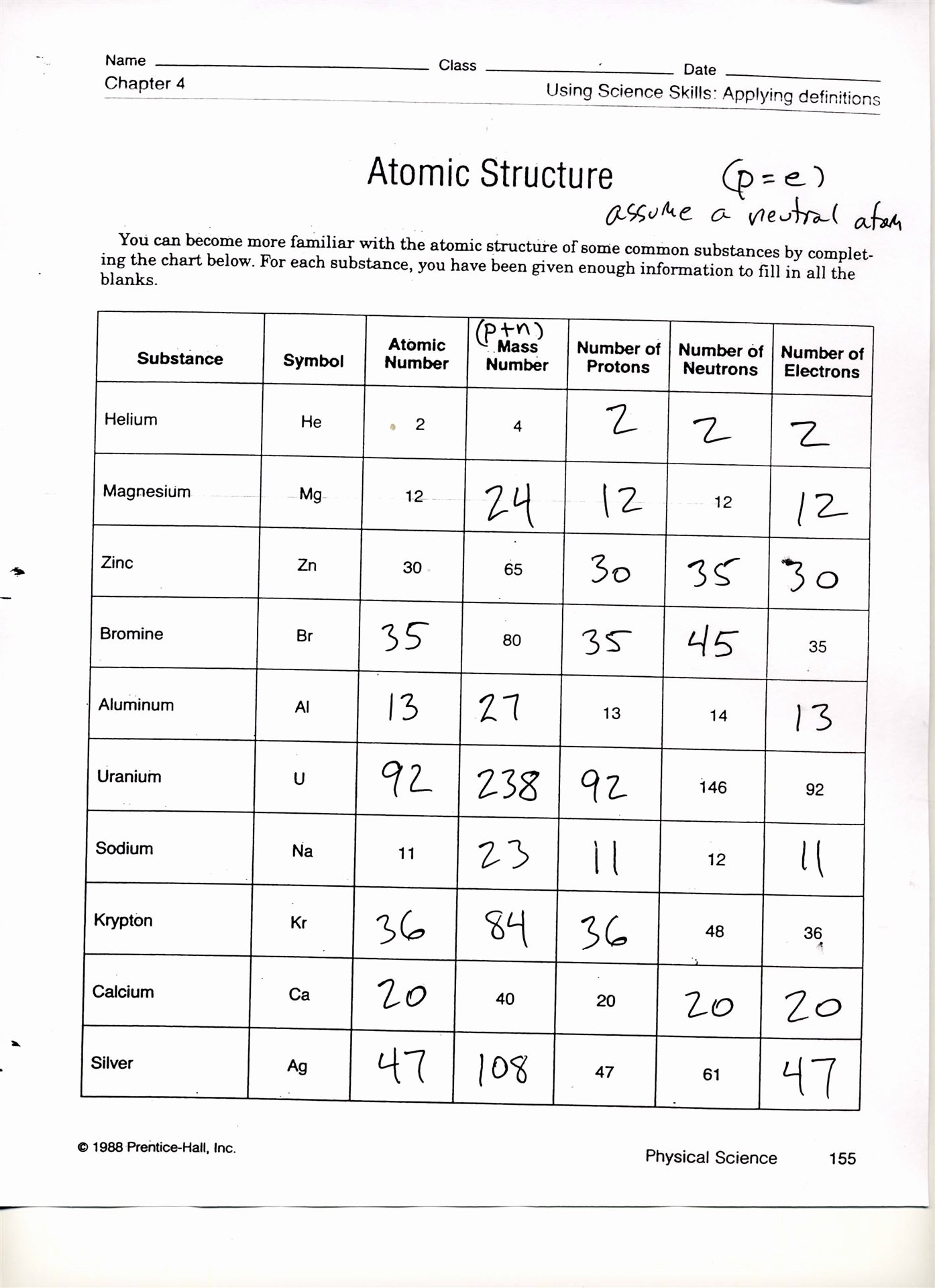
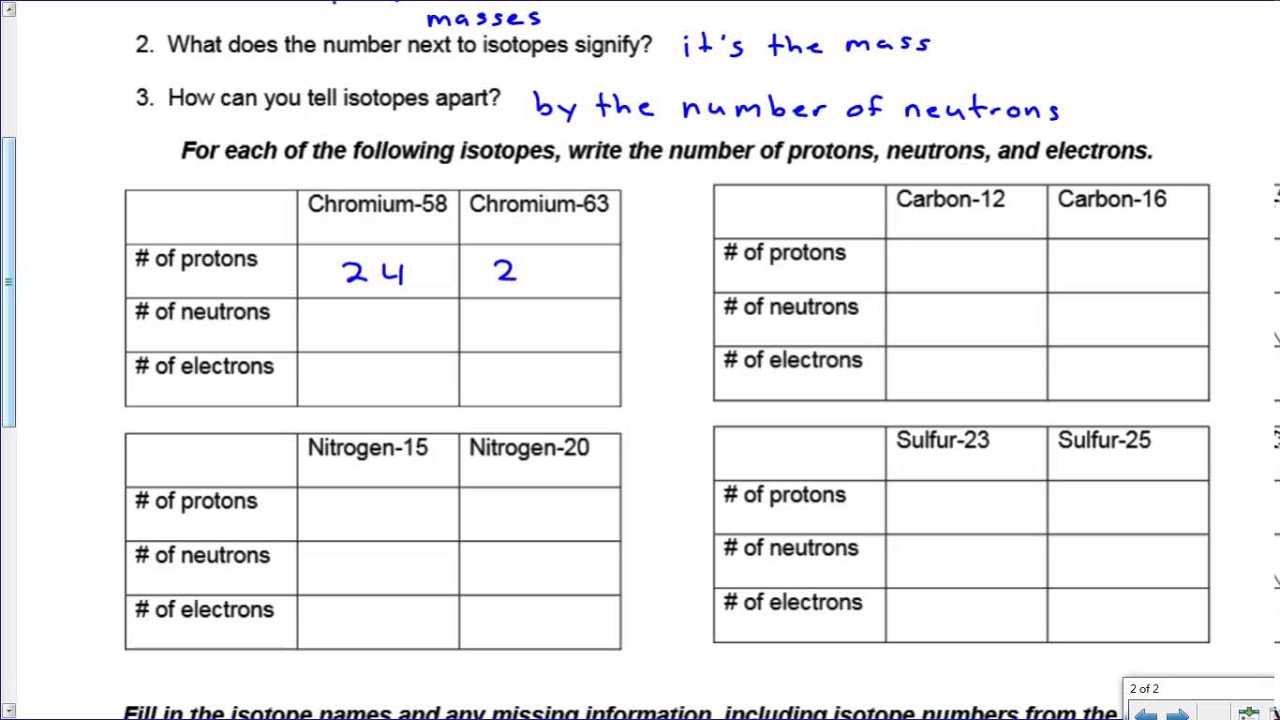
Isotope Practice Sheet
Isotope Practice Sheet - This set of worksheets is designed to explore the fundamental concept of isotopes and their significance in chemistry. Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), 87 (abundance of 7.0%), and 88 (abundance of. Fill in the isotope names and any missing information, including. A common way of writing the name of an isotope is to put the mass number after the name, for the isotopes above the names would be; This worksheet provides practice with isotopes, a fundamental concept in chemistry. This quiz helps you practice naming, determining notation, and counting subatomic particles for hundreds of elements and isotopes. It includes problems to help students calculate atomic. Here are three isotopes of an element: For each of the following isotopes, write the # of protons, neutrons, and electrons. The number 6 refers to the atomic number.
Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), 87 (abundance of 7.0%), and 88 (abundance of. This worksheet provides practice with isotopes, a fundamental concept in chemistry. A common way of writing the name of an isotope is to put the mass number after the name, for the isotopes above the names would be; The number 6 refers to the atomic number. For each of the following isotopes, write the # of protons, neutrons, and electrons. Fill in the isotope names and any missing information, including. This set of worksheets is designed to explore the fundamental concept of isotopes and their significance in chemistry. It includes problems to help students calculate atomic. Here are three isotopes of an element: This quiz helps you practice naming, determining notation, and counting subatomic particles for hundreds of elements and isotopes.
This quiz helps you practice naming, determining notation, and counting subatomic particles for hundreds of elements and isotopes. For each of the following isotopes, write the # of protons, neutrons, and electrons. This worksheet provides practice with isotopes, a fundamental concept in chemistry. It includes problems to help students calculate atomic. Fill in the isotope names and any missing information, including. Here are three isotopes of an element: A common way of writing the name of an isotope is to put the mass number after the name, for the isotopes above the names would be; Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), 87 (abundance of 7.0%), and 88 (abundance of. The number 6 refers to the atomic number. This set of worksheets is designed to explore the fundamental concept of isotopes and their significance in chemistry.
Practice Isotope Calculations 1 Worksheet Answers
Fill in the isotope names and any missing information, including. This set of worksheets is designed to explore the fundamental concept of isotopes and their significance in chemistry. For each of the following isotopes, write the # of protons, neutrons, and electrons. Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), 87 (abundance of.
Isotope Worksheet
Here are three isotopes of an element: The number 6 refers to the atomic number. This set of worksheets is designed to explore the fundamental concept of isotopes and their significance in chemistry. Fill in the isotope names and any missing information, including. It includes problems to help students calculate atomic.
Isotope Worksheet
A common way of writing the name of an isotope is to put the mass number after the name, for the isotopes above the names would be; Fill in the isotope names and any missing information, including. This quiz helps you practice naming, determining notation, and counting subatomic particles for hundreds of elements and isotopes. Here are three isotopes of.
Free Printable Isotope Practice Worksheets for Students
This quiz helps you practice naming, determining notation, and counting subatomic particles for hundreds of elements and isotopes. Here are three isotopes of an element: It includes problems to help students calculate atomic. A common way of writing the name of an isotope is to put the mass number after the name, for the isotopes above the names would be;.
Isotope Practice Worksheet Isotopes And Ions Worksheet
It includes problems to help students calculate atomic. This set of worksheets is designed to explore the fundamental concept of isotopes and their significance in chemistry. Fill in the isotope names and any missing information, including. This worksheet provides practice with isotopes, a fundamental concept in chemistry. Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance.
50 isotope Practice Worksheet Answer Key Chessmuseum Template Library
This worksheet provides practice with isotopes, a fundamental concept in chemistry. A common way of writing the name of an isotope is to put the mass number after the name, for the isotopes above the names would be; This set of worksheets is designed to explore the fundamental concept of isotopes and their significance in chemistry. Strontium consists of four.
Isotope And Ions Practice Worksheet
The number 6 refers to the atomic number. Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), 87 (abundance of 7.0%), and 88 (abundance of. A common way of writing the name of an isotope is to put the mass number after the name, for the isotopes above the names would be; Fill in.
isotope practice worksheet answers atoms ions and isotopes worksheet
This worksheet provides practice with isotopes, a fundamental concept in chemistry. The number 6 refers to the atomic number. A common way of writing the name of an isotope is to put the mass number after the name, for the isotopes above the names would be; It includes problems to help students calculate atomic. This quiz helps you practice naming,.
Isotope Notation Worksheet
Fill in the isotope names and any missing information, including. This worksheet provides practice with isotopes, a fundamental concept in chemistry. This quiz helps you practice naming, determining notation, and counting subatomic particles for hundreds of elements and isotopes. For each of the following isotopes, write the # of protons, neutrons, and electrons. This set of worksheets is designed to.
Free Printable Isotope Practice Worksheets for Students
For each of the following isotopes, write the # of protons, neutrons, and electrons. This worksheet provides practice with isotopes, a fundamental concept in chemistry. A common way of writing the name of an isotope is to put the mass number after the name, for the isotopes above the names would be; Here are three isotopes of an element: This.
This Set Of Worksheets Is Designed To Explore The Fundamental Concept Of Isotopes And Their Significance In Chemistry.
For each of the following isotopes, write the # of protons, neutrons, and electrons. Fill in the isotope names and any missing information, including. The number 6 refers to the atomic number. This worksheet provides practice with isotopes, a fundamental concept in chemistry.
Here Are Three Isotopes Of An Element:
Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), 87 (abundance of 7.0%), and 88 (abundance of. It includes problems to help students calculate atomic. A common way of writing the name of an isotope is to put the mass number after the name, for the isotopes above the names would be; This quiz helps you practice naming, determining notation, and counting subatomic particles for hundreds of elements and isotopes.