When Did Prevnar 20 Become Available
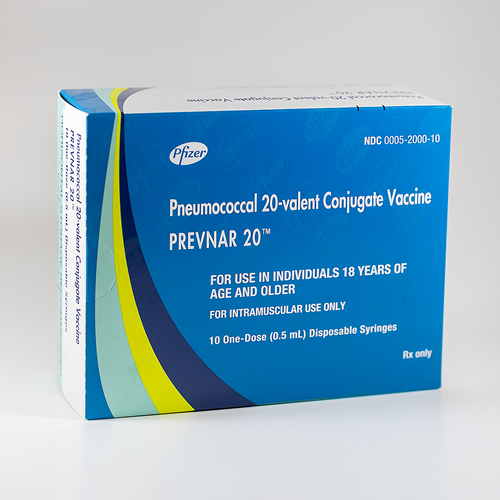
When Did Prevnar 20 Become Available - In april 2023, the fda approved prevnar 20 for the prevention of invasive disease caused by the 20 different serotypes of s. Active immunization for the prevention of otitis media caused by s. Pneumoniae serotypes 4, 6b, 9v, 14, 18c, 19f,.
Active immunization for the prevention of otitis media caused by s. Pneumoniae serotypes 4, 6b, 9v, 14, 18c, 19f,. In april 2023, the fda approved prevnar 20 for the prevention of invasive disease caused by the 20 different serotypes of s.
Pneumoniae serotypes 4, 6b, 9v, 14, 18c, 19f,. Active immunization for the prevention of otitis media caused by s. In april 2023, the fda approved prevnar 20 for the prevention of invasive disease caused by the 20 different serotypes of s.
Did You Know? New Pneumococcal Vaccination
Pneumoniae serotypes 4, 6b, 9v, 14, 18c, 19f,. In april 2023, the fda approved prevnar 20 for the prevention of invasive disease caused by the 20 different serotypes of s. Active immunization for the prevention of otitis media caused by s.
Zydus Cadila India approves world's first DNA Covid vaccine BBC News
Active immunization for the prevention of otitis media caused by s. Pneumoniae serotypes 4, 6b, 9v, 14, 18c, 19f,. In april 2023, the fda approved prevnar 20 for the prevention of invasive disease caused by the 20 different serotypes of s.
Prevnar 20 TV Spot, 'Keep My Plans Getting Vaccinated' iSpot
Active immunization for the prevention of otitis media caused by s. In april 2023, the fda approved prevnar 20 for the prevention of invasive disease caused by the 20 different serotypes of s. Pneumoniae serotypes 4, 6b, 9v, 14, 18c, 19f,.
ACIP Endorses 20Valent Pneumococcal Vaccine for Kids MedPage Today
Active immunization for the prevention of otitis media caused by s. Pneumoniae serotypes 4, 6b, 9v, 14, 18c, 19f,. In april 2023, the fda approved prevnar 20 for the prevention of invasive disease caused by the 20 different serotypes of s.
Promising results for COVID19 and pneumococcal vaccine coadministration
Active immunization for the prevention of otitis media caused by s. Pneumoniae serotypes 4, 6b, 9v, 14, 18c, 19f,. In april 2023, the fda approved prevnar 20 for the prevention of invasive disease caused by the 20 different serotypes of s.
20Valent Pneumococcal Vax Adequate for Older Adults MedPage Today
In april 2023, the fda approved prevnar 20 for the prevention of invasive disease caused by the 20 different serotypes of s. Pneumoniae serotypes 4, 6b, 9v, 14, 18c, 19f,. Active immunization for the prevention of otitis media caused by s.
Prevnar 20 Pneumococcal 20valent Conjugate Vaccine), suspension for
Active immunization for the prevention of otitis media caused by s. Pneumoniae serotypes 4, 6b, 9v, 14, 18c, 19f,. In april 2023, the fda approved prevnar 20 for the prevention of invasive disease caused by the 20 different serotypes of s.
DailyMed PREVNAR 20 pneumococcal 20valent conjugate vaccine
Pneumoniae serotypes 4, 6b, 9v, 14, 18c, 19f,. In april 2023, the fda approved prevnar 20 for the prevention of invasive disease caused by the 20 different serotypes of s. Active immunization for the prevention of otitis media caused by s.
RSV vaccine CDC panel shots for American adults 65 and up
Pneumoniae serotypes 4, 6b, 9v, 14, 18c, 19f,. In april 2023, the fda approved prevnar 20 for the prevention of invasive disease caused by the 20 different serotypes of s. Active immunization for the prevention of otitis media caused by s.
Prevnar 20 Pneumococcal 20valent Conjugate Vaccine [Diphtheria CRM197
Active immunization for the prevention of otitis media caused by s. In april 2023, the fda approved prevnar 20 for the prevention of invasive disease caused by the 20 different serotypes of s. Pneumoniae serotypes 4, 6b, 9v, 14, 18c, 19f,.
In April 2023, The Fda Approved Prevnar 20 For The Prevention Of Invasive Disease Caused By The 20 Different Serotypes Of S.
Active immunization for the prevention of otitis media caused by s. Pneumoniae serotypes 4, 6b, 9v, 14, 18c, 19f,.