When Will Lepodisiran Be Available
When Will Lepodisiran Be Available - Lepodisiran sodium is under clinical development by eli lilly and co and currently in phase iii for atherosclerosis. Lepodisiran is not currently available in the united states. This medication, which is an investigational rna interference (rnai). Lepodisiran is an investigational sirna therapy for adults with elevated lp (a), a genetic risk factor for heart disease.
Lepodisiran is an investigational sirna therapy for adults with elevated lp (a), a genetic risk factor for heart disease. This medication, which is an investigational rna interference (rnai). Lepodisiran sodium is under clinical development by eli lilly and co and currently in phase iii for atherosclerosis. Lepodisiran is not currently available in the united states.
This medication, which is an investigational rna interference (rnai). Lepodisiran is an investigational sirna therapy for adults with elevated lp (a), a genetic risk factor for heart disease. Lepodisiran sodium is under clinical development by eli lilly and co and currently in phase iii for atherosclerosis. Lepodisiran is not currently available in the united states.
Encouraging data for lepodisiran as Lp(a) lowering therapy Medical
Lepodisiran is not currently available in the united states. Lepodisiran sodium is under clinical development by eli lilly and co and currently in phase iii for atherosclerosis. Lepodisiran is an investigational sirna therapy for adults with elevated lp (a), a genetic risk factor for heart disease. This medication, which is an investigational rna interference (rnai).
Lepodisiran, an ExtendedDuration Short Interfering RNA Targeting
Lepodisiran sodium is under clinical development by eli lilly and co and currently in phase iii for atherosclerosis. Lepodisiran is an investigational sirna therapy for adults with elevated lp (a), a genetic risk factor for heart disease. Lepodisiran is not currently available in the united states. This medication, which is an investigational rna interference (rnai).
Experimental drug cuts heart disease risk factor by 96 Big Think
Lepodisiran is not currently available in the united states. This medication, which is an investigational rna interference (rnai). Lepodisiran is an investigational sirna therapy for adults with elevated lp (a), a genetic risk factor for heart disease. Lepodisiran sodium is under clinical development by eli lilly and co and currently in phase iii for atherosclerosis.
前沿速递JAMALepodisiran,一种靶向脂蛋白(a)的长效短干扰RNA:剂量递增的随机…_澎湃号·政务_澎湃新闻The Paper
This medication, which is an investigational rna interference (rnai). Lepodisiran sodium is under clinical development by eli lilly and co and currently in phase iii for atherosclerosis. Lepodisiran is an investigational sirna therapy for adults with elevated lp (a), a genetic risk factor for heart disease. Lepodisiran is not currently available in the united states.
Lepodisiran, an ExtendedDuration Short Interfering RNA Targeting
Lepodisiran sodium is under clinical development by eli lilly and co and currently in phase iii for atherosclerosis. Lepodisiran is not currently available in the united states. This medication, which is an investigational rna interference (rnai). Lepodisiran is an investigational sirna therapy for adults with elevated lp (a), a genetic risk factor for heart disease.
Lepodisiran An ExtendedDuration siRNA Targeting Lipoprotein(a
This medication, which is an investigational rna interference (rnai). Lepodisiran is not currently available in the united states. Lepodisiran sodium is under clinical development by eli lilly and co and currently in phase iii for atherosclerosis. Lepodisiran is an investigational sirna therapy for adults with elevated lp (a), a genetic risk factor for heart disease.
Lepodisiran Sodium for Elevated Lipoprotein Clinical Trial 2024 Power
Lepodisiran is not currently available in the united states. Lepodisiran is an investigational sirna therapy for adults with elevated lp (a), a genetic risk factor for heart disease. Lepodisiran sodium is under clinical development by eli lilly and co and currently in phase iii for atherosclerosis. This medication, which is an investigational rna interference (rnai).
Large, Sustained Reductions in Lp(a) With Lepodisiran in Phase I Study
Lepodisiran is not currently available in the united states. Lepodisiran is an investigational sirna therapy for adults with elevated lp (a), a genetic risk factor for heart disease. Lepodisiran sodium is under clinical development by eli lilly and co and currently in phase iii for atherosclerosis. This medication, which is an investigational rna interference (rnai).
Jama nissen 2023 oi 230131 1701196141 Lepodisiran, an Extended
This medication, which is an investigational rna interference (rnai). Lepodisiran is an investigational sirna therapy for adults with elevated lp (a), a genetic risk factor for heart disease. Lepodisiran is not currently available in the united states. Lepodisiran sodium is under clinical development by eli lilly and co and currently in phase iii for atherosclerosis.
Single dose of lepodisiran reduces lipoprotein A more than 94 percent
Lepodisiran sodium is under clinical development by eli lilly and co and currently in phase iii for atherosclerosis. Lepodisiran is an investigational sirna therapy for adults with elevated lp (a), a genetic risk factor for heart disease. Lepodisiran is not currently available in the united states. This medication, which is an investigational rna interference (rnai).
Lepodisiran Is An Investigational Sirna Therapy For Adults With Elevated Lp (A), A Genetic Risk Factor For Heart Disease.
This medication, which is an investigational rna interference (rnai). Lepodisiran is not currently available in the united states. Lepodisiran sodium is under clinical development by eli lilly and co and currently in phase iii for atherosclerosis.



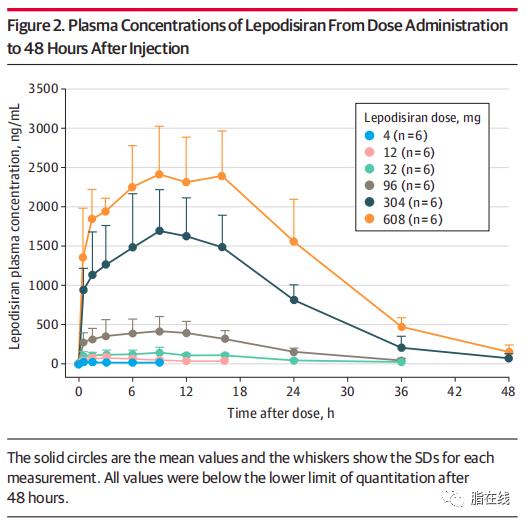


, Elevated Lp(a):%0A%0ALepodisiran Sodium for Elevated Lipoprotein.png?md=1)
 With Lepodisiran in Phase I Study HEADER.jpg)