When Will Roflumilast Foam Be Available
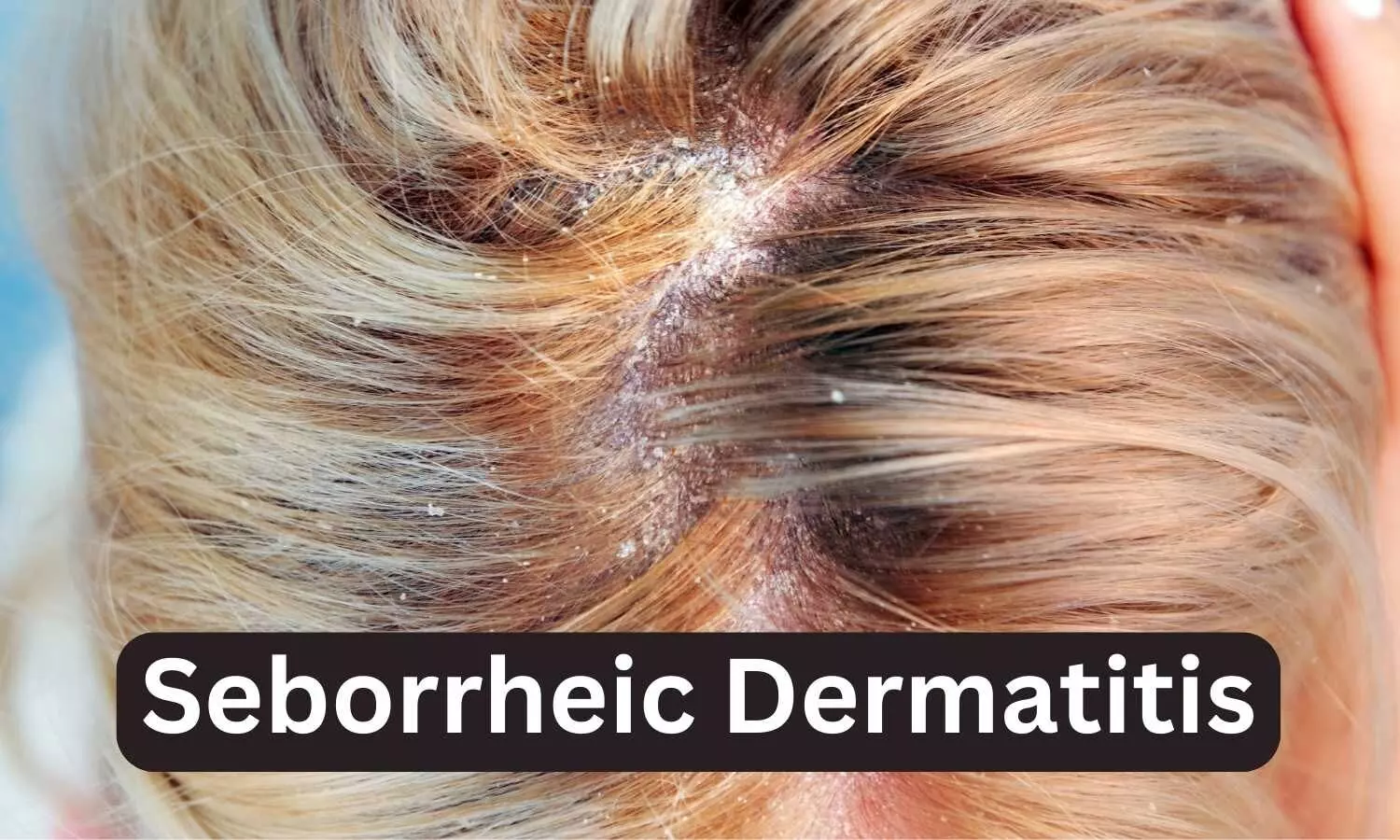
When Will Roflumilast Foam Be Available - Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the treatment of. On may 22, 2025, the fda approved roflumilast (zoryve; Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key.
Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. On may 22, 2025, the fda approved roflumilast (zoryve; Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the treatment of. Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and.
On may 22, 2025, the fda approved roflumilast (zoryve; Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the treatment of.
FDA Approves Arcutis’ ZORYVE® (roflumilast) Topical Foam, 0.3 for the
Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. On may 22, 2025, the fda approved roflumilast (zoryve; The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. Arcutis.
ZORYVE (roflumilast) topical foam 0.3 Official HCP Website
The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. On may 22, 2025, the fda approved roflumilast (zoryve; Arcutis.
ZORYVE® (roflumilast) topical foam, 0.3 Patient Website
On may 22, 2025, the fda approved roflumilast (zoryve; The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the.
Roflumilast
The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the treatment of. On may 22, 2025, the fda approved roflumilast (zoryve; Arcutis intends to make.
Roflumilast Tablets Solco Healthcare
Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. On may 22, 2025, the fda approved roflumilast (zoryve; Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. The.
ZORYVE® (roflumilast) topical foam, 0.3 Arcutis Biotherapeutics
Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. Arcutis biotherapeutics) topical foam 0.3%.
FDA Approves Arcutis’ ZORYVE® (roflumilast) Cream 0.3 for Treatment of
Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. On may 22, 2025, the fda approved roflumilast (zoryve; Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the.
Roflumilast topical foam receives FDA approval for treating seborrheic
Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. On may 22, 2025, the fda approved roflumilast (zoryve; Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy.
DailyMed ZORYVE roflumilast aerosol, foam
The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. Food and drug administration has approved zoryve (roflumilast) topical foam 0.3 percent for the treatment of. Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new. On may 22, 2025, the fda approved roflumilast.
ZORYVE® (roflumilast) topical foam, 0.3 Patient Website
The 0.3% foam formulation of roflumilast was previously approved for the treatment of seborrheic dermatitis in adults and. Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. Arcutis intends to make zoryve foam widely available via key.
Food And Drug Administration Has Approved Zoryve (Roflumilast) Topical Foam 0.3 Percent For The Treatment Of.
On may 22, 2025, the fda approved roflumilast (zoryve; Zoryve foam 0.3% is also approved for the treatment of seborrheic dermatitis and is widely available today via key. Arcutis biotherapeutics) topical foam 0.3% to treat plaque psoriasis of the scalp and. Arcutis intends to make zoryve foam widely available via key wholesaler and dermatology pharmacy channels as a new.