When Will Trodusquemine Be Available Usa
When Will Trodusquemine Be Available Usa - A ptp1b inhibitors drug, initially developed by depymed, inc., now, its global highest r&d status is preclinical,.
A ptp1b inhibitors drug, initially developed by depymed, inc., now, its global highest r&d status is preclinical,.
A ptp1b inhibitors drug, initially developed by depymed, inc., now, its global highest r&d status is preclinical,.
Trodusquemine Availability and Approval Timeline
A ptp1b inhibitors drug, initially developed by depymed, inc., now, its global highest r&d status is preclinical,.
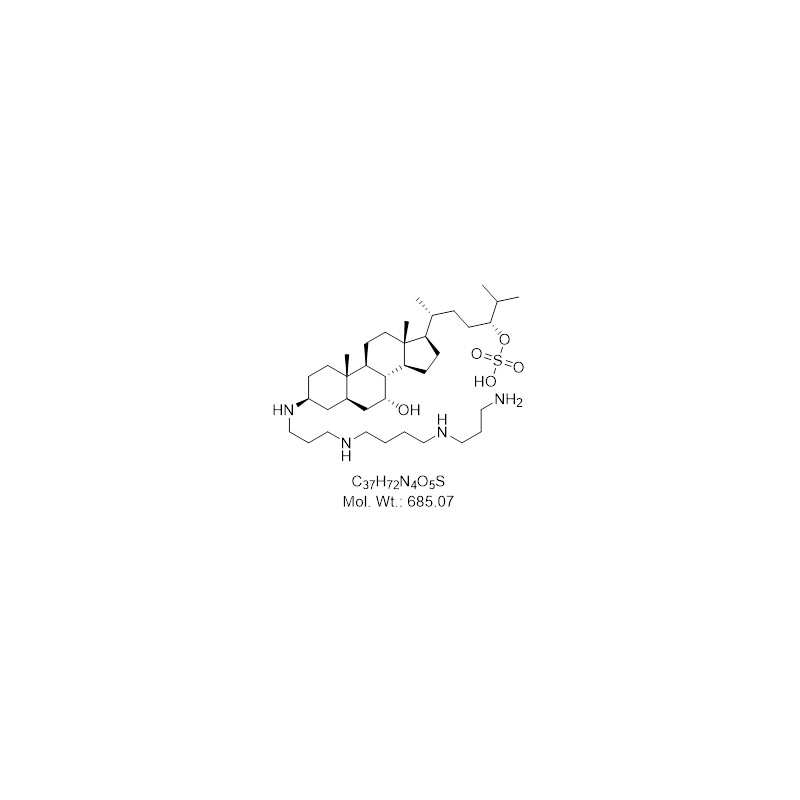
Trodusquemine (MSI1436) supplier CAS 186139093 PTP1B Inhibitor
A ptp1b inhibitors drug, initially developed by depymed, inc., now, its global highest r&d status is preclinical,.
Squalamine and trodusquemine two natural products for
A ptp1b inhibitors drug, initially developed by depymed, inc., now, its global highest r&d status is preclinical,.
Trodusquemine Availability and Approval Timeline
A ptp1b inhibitors drug, initially developed by depymed, inc., now, its global highest r&d status is preclinical,.
Squalamine and trodusquemine two natural products for
A ptp1b inhibitors drug, initially developed by depymed, inc., now, its global highest r&d status is preclinical,.
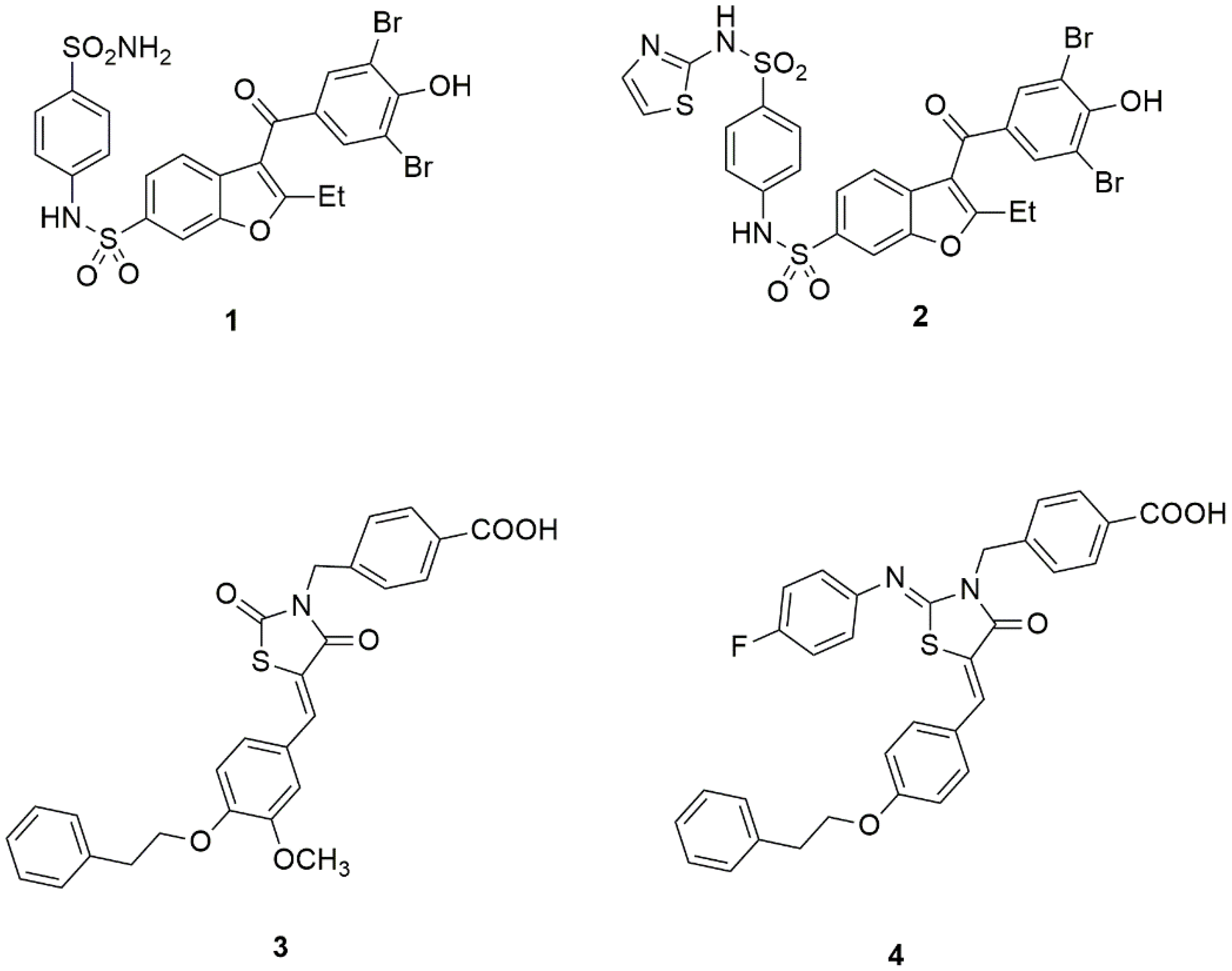
IJMS Free FullText Can Allostery Be a Key Strategy for Targeting
A ptp1b inhibitors drug, initially developed by depymed, inc., now, its global highest r&d status is preclinical,.
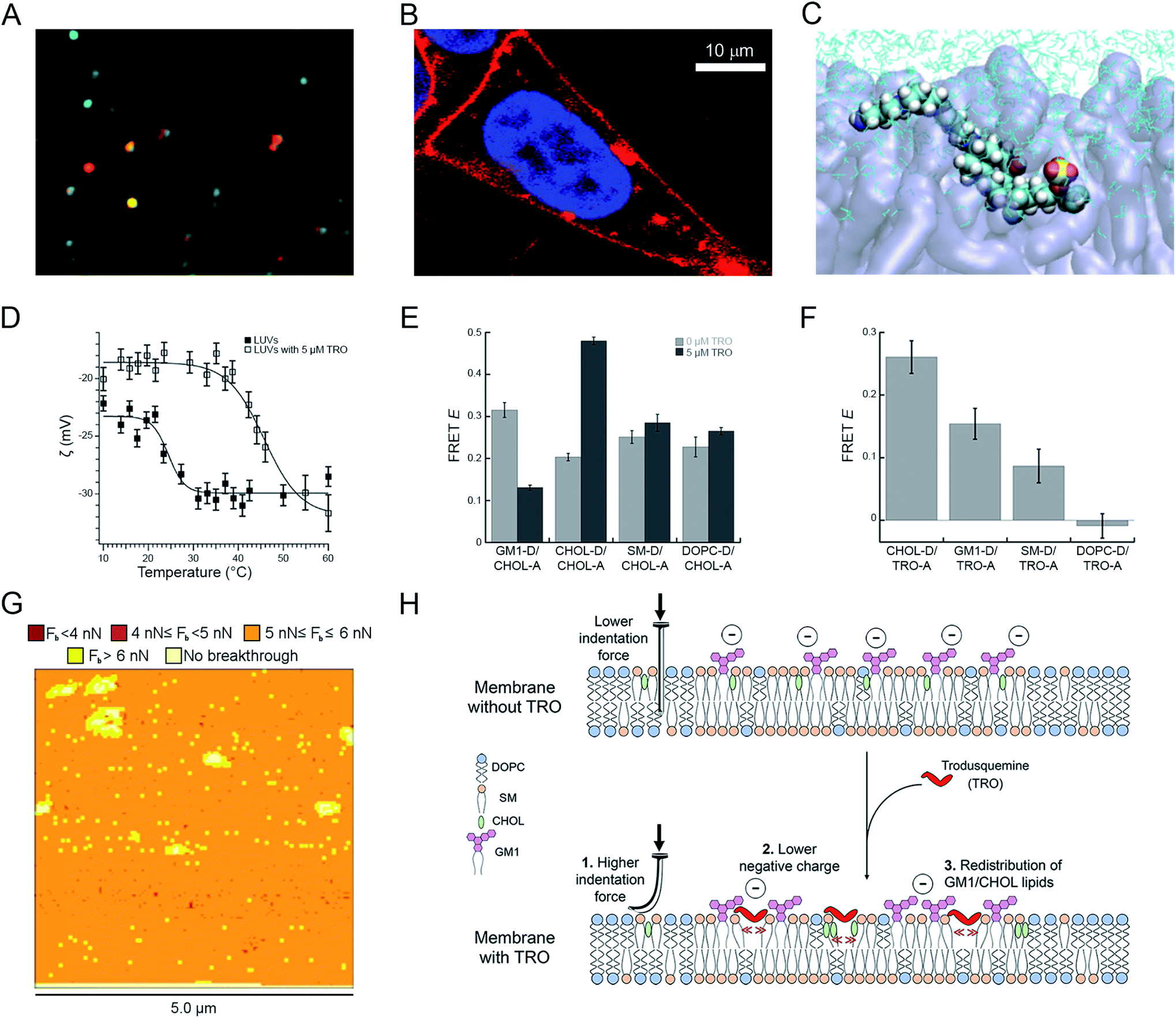
Central Trodusquemine administration restored the response to insulin
A ptp1b inhibitors drug, initially developed by depymed, inc., now, its global highest r&d status is preclinical,.
Squalamine and trodusquemine two natural products for
A ptp1b inhibitors drug, initially developed by depymed, inc., now, its global highest r&d status is preclinical,.
MSI1436C (Trodusquemine) 20 mg/m2 IV for Malignant Neoplasms Clinical
A ptp1b inhibitors drug, initially developed by depymed, inc., now, its global highest r&d status is preclinical,.




 20 mg/m2 IV for Malignant Neoplasms.png?md=1)
