When Will Vx-548 Be Available
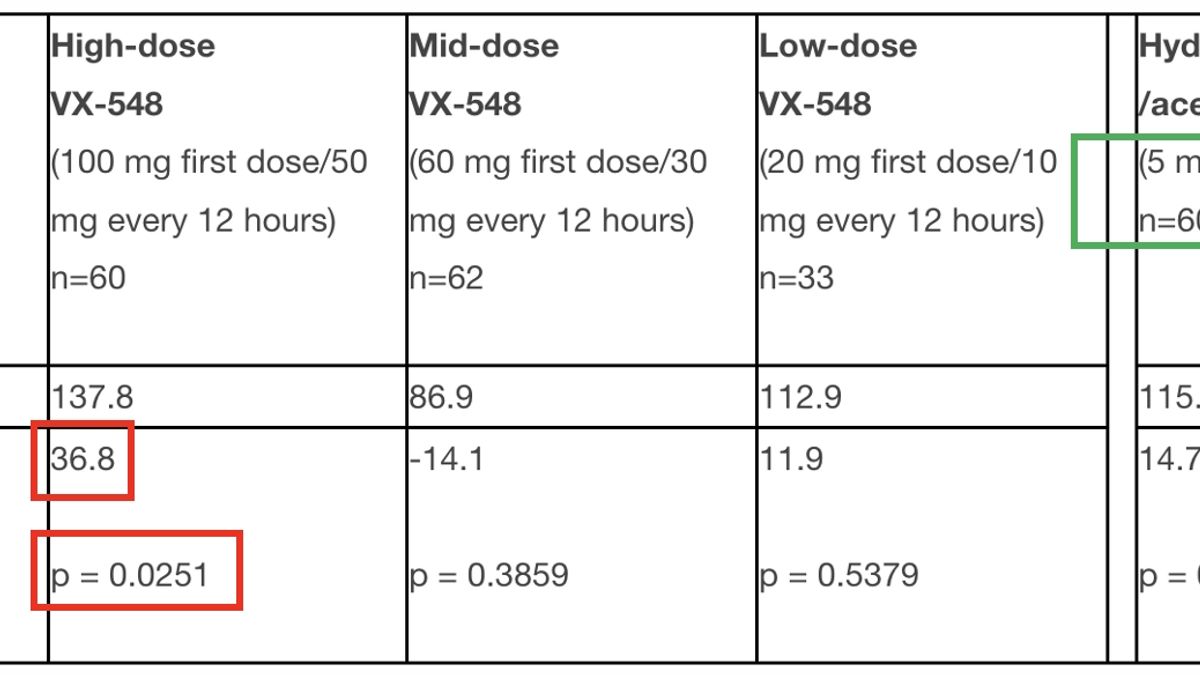
When Will Vx-548 Be Available - Fda has just accepted vertex’s new drug application related to suzetrigine for priority review in late january, 2025. Following the positive phase 3 results in acute pain. Key inclusion criteria in rcts:. Journavx (suzetrigine) is a sodium channel blocker indicated for the treatment of moderate to severe acute pain in adults.
Key inclusion criteria in rcts:. Following the positive phase 3 results in acute pain. Journavx (suzetrigine) is a sodium channel blocker indicated for the treatment of moderate to severe acute pain in adults. Fda has just accepted vertex’s new drug application related to suzetrigine for priority review in late january, 2025.
Key inclusion criteria in rcts:. Journavx (suzetrigine) is a sodium channel blocker indicated for the treatment of moderate to severe acute pain in adults. Following the positive phase 3 results in acute pain. Fda has just accepted vertex’s new drug application related to suzetrigine for priority review in late january, 2025.
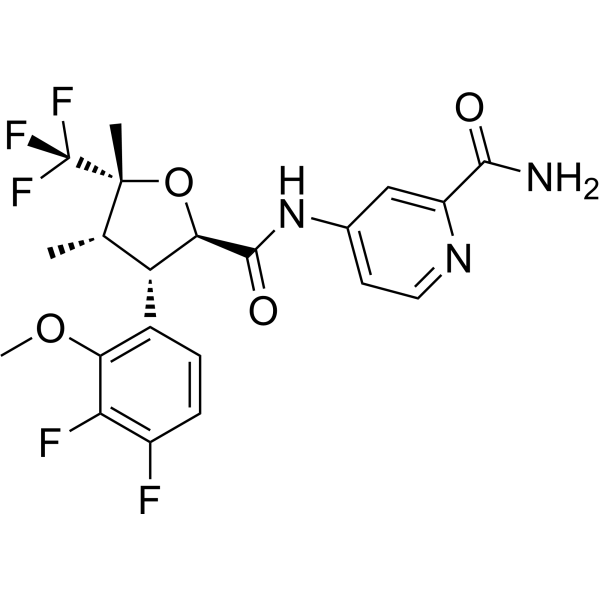
suzetrigine (VX548)
Journavx (suzetrigine) is a sodium channel blocker indicated for the treatment of moderate to severe acute pain in adults. Following the positive phase 3 results in acute pain. Key inclusion criteria in rcts:. Fda has just accepted vertex’s new drug application related to suzetrigine for priority review in late january, 2025.
VX548 for Peripheral Neuropathy Clinical Trial 2024 Power
Following the positive phase 3 results in acute pain. Key inclusion criteria in rcts:. Fda has just accepted vertex’s new drug application related to suzetrigine for priority review in late january, 2025. Journavx (suzetrigine) is a sodium channel blocker indicated for the treatment of moderate to severe acute pain in adults.
Selective Inhibition of NaV1.8 with VX548 for Acute Pain NEJM
Following the positive phase 3 results in acute pain. Key inclusion criteria in rcts:. Journavx (suzetrigine) is a sodium channel blocker indicated for the treatment of moderate to severe acute pain in adults. Fda has just accepted vertex’s new drug application related to suzetrigine for priority review in late january, 2025.
Selective Inhibition of NaV1.8 with VX548 for Acute Pain New England
Fda has just accepted vertex’s new drug application related to suzetrigine for priority review in late january, 2025. Journavx (suzetrigine) is a sodium channel blocker indicated for the treatment of moderate to severe acute pain in adults. Key inclusion criteria in rcts:. Following the positive phase 3 results in acute pain.
Suzetrigine (VX548) Sodium Channel Blocker, NaV1.8 Inhibitor
Following the positive phase 3 results in acute pain. Journavx (suzetrigine) is a sodium channel blocker indicated for the treatment of moderate to severe acute pain in adults. Fda has just accepted vertex’s new drug application related to suzetrigine for priority review in late january, 2025. Key inclusion criteria in rcts:.
Selective Inhibition of NaV1.8 with VX548 for Acute Pain NEJM
Key inclusion criteria in rcts:. Following the positive phase 3 results in acute pain. Fda has just accepted vertex’s new drug application related to suzetrigine for priority review in late january, 2025. Journavx (suzetrigine) is a sodium channel blocker indicated for the treatment of moderate to severe acute pain in adults.
Selective Inhibition of NaV1.8 with VX548 for Acute Pain New England
Fda has just accepted vertex’s new drug application related to suzetrigine for priority review in late january, 2025. Journavx (suzetrigine) is a sodium channel blocker indicated for the treatment of moderate to severe acute pain in adults. Key inclusion criteria in rcts:. Following the positive phase 3 results in acute pain.
Vertex's Pain Medicine VX548 A New Hope for NonOpioid Pain Relief
Key inclusion criteria in rcts:. Following the positive phase 3 results in acute pain. Journavx (suzetrigine) is a sodium channel blocker indicated for the treatment of moderate to severe acute pain in adults. Fda has just accepted vertex’s new drug application related to suzetrigine for priority review in late january, 2025.
Selective Inhibition of NaV1.8 with VX548 for Acute Pain New England
Key inclusion criteria in rcts:. Fda has just accepted vertex’s new drug application related to suzetrigine for priority review in late january, 2025. Journavx (suzetrigine) is a sodium channel blocker indicated for the treatment of moderate to severe acute pain in adults. Following the positive phase 3 results in acute pain.
VX548 for Pain Clinical Trial 2024 Power
Following the positive phase 3 results in acute pain. Key inclusion criteria in rcts:. Journavx (suzetrigine) is a sodium channel blocker indicated for the treatment of moderate to severe acute pain in adults. Fda has just accepted vertex’s new drug application related to suzetrigine for priority review in late january, 2025.
Key Inclusion Criteria In Rcts:.
Following the positive phase 3 results in acute pain. Journavx (suzetrigine) is a sodium channel blocker indicated for the treatment of moderate to severe acute pain in adults. Fda has just accepted vertex’s new drug application related to suzetrigine for priority review in late january, 2025.
:%0A%0AVX-548 for Peripheral Neuropathy.png?md=1)